BPDF Aids Pharma Company with
COVID-19 Vaccine Development
COVID-19 Vaccine Development
The BPDF is working with Vault Pharma, an emerging biotechnology company, on a vaccine that could treat patients who have the novel coronavirus and help prevent similar outbreaks in the future. The vaccine could be a complementary weapon to any initial vaccine for COVID-19 treatment. READ MORE
BPDF Producing Protein Therapeutic
for Coronavirus Patients
for Coronavirus Patients
Working with a preclinical drug discovery company using its proprietary genetic technology platform, the BPDF is in early-stage production of a recombinant protein as a potential biotherapeutic for COVID-19 patients who may have few options for treatment.
READ MORE
READ MORE
The Biological Process Development Facility (BPDF) at the University of Nebraska offers biopharmaceutical process development and biomanufacturing services that transition discoveries into early phase clinical trials. BPDF capabilities include:
- Master and working cell banks
- Upstream and downstream process development
- Stability testing services
- Analytical method development and qualification
- Microbial manufacture of biologics
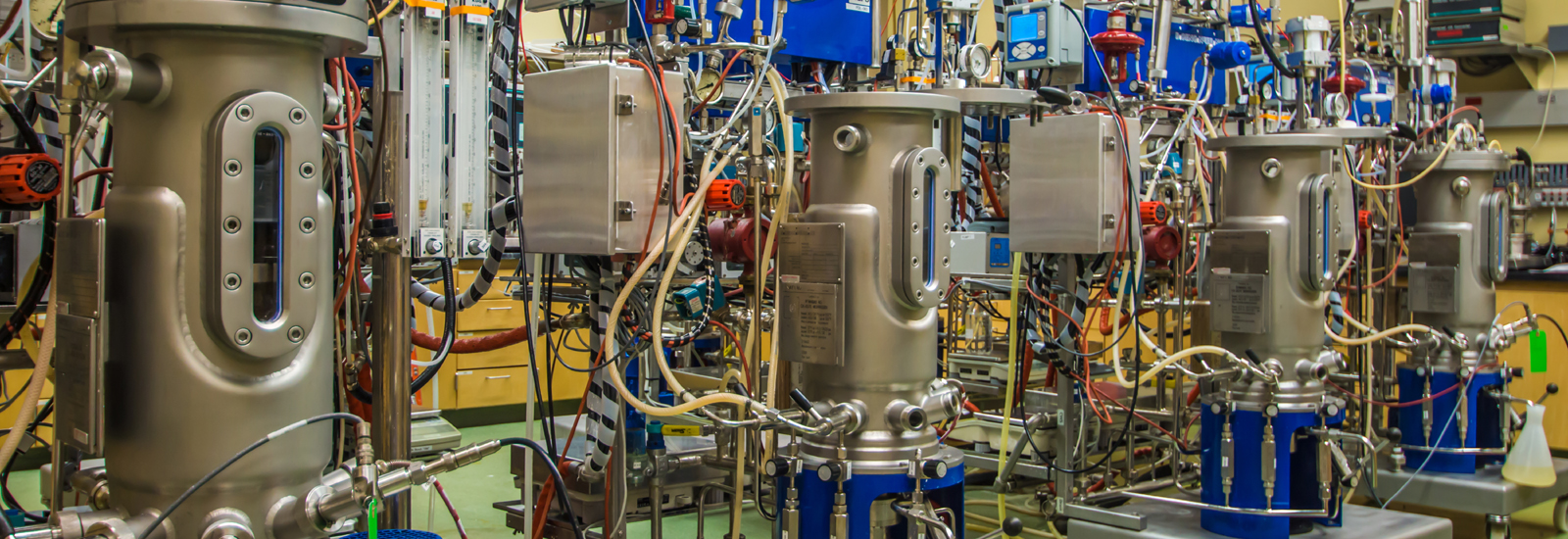
Fermentation processes focus on optimizing and controlling high cell-density fermentations of Pichia pastoris, Saccharomyces cerevisiae, and Escherichia coli for recombinant protein production. Activities are governed by a rigorous quality system as well as regulatory support.
The BPDF has produced a wide range of biologics—including vaccines, recombinant proteins, gene therapies and other biotherapeutics—in partnership with government agencies, biotechnology companies, academic researchers, and non-profit organizations. The BPDF applies decades of expertise and experience to developing scalable and reproducible cGMP manufacturing processes.