 The Biological Process Development Facility (BPDF) recognizes that each project is unique, and therefore tailors its approach to meet the specific needs and goals of the project and the client. Our project team integrates process, quality, and business considerations to provide clients with a seamless experience in addressing the challenges that arise during the design, development, and production of biopharmaceuticals and other proprietary proteins and products.
The Biological Process Development Facility (BPDF) recognizes that each project is unique, and therefore tailors its approach to meet the specific needs and goals of the project and the client. Our project team integrates process, quality, and business considerations to provide clients with a seamless experience in addressing the challenges that arise during the design, development, and production of biopharmaceuticals and other proprietary proteins and products.
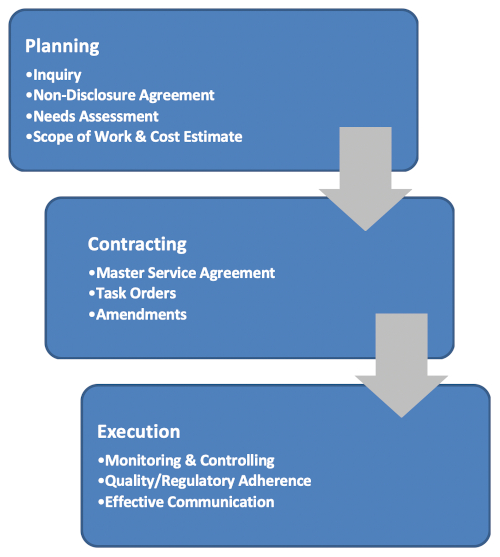
Our rigorous planning process lays a solid foundation for a successful project. Beginning with an initial inquiry, discussion, and needs assessment, we determine whether the BPDF has the capacity and expertise to fulfill the project's objectives. Initially, a non-disclosure agreement (NDA) is put in place to allow the client and BPDF to share critical information needed to construct an initial cost estimate and ultimately a Scope of Work. If a decision is made to move forward, we negotiate a Master Service Agreement (MSA), which allows both parties to agree to the general business rules of engagement and enables a more agile response to issuing individual task orders to keep project progress moving forward.
During a project's execution, our team closely monitors and controls project activities, making certain to align scope, schedule, and product specifications with client expectations and budget. Our Quality Systems Group ensures that Good Documentation, Laboratory, and Manufacturing Practices are adhered to during each phase of a project.
We understand that timely, accurate, and concise communication is critical during the execution of a project. Our team ensures that effective communication and data sharing tools and strategies are in place to enable you to make informed and timely decisions. We offer on-site visits and facility tours during initial contact, planning, and production phases.
If you are interested in working with the BPDF, we encourage you to fill out our Project Inquiry Form, or contact us by phone at 402-472-1983.