In a pair of announcements this month, the Environmental Protection Agency (EPA) set forth new rules and standards regarding the levels of “forever chemicals” in America’s drinking water.
These PFAS (per- and polyfluoroalkyl substances), which have been linked to cancer and other health issues, are found in most consumer products that include Teflon, other non-stick cooking pans, food packaging and water-repellant coatings.
A research team headed by Nebraska Engineering’s Nirupam Aich believes it has found a way to use nanomaterials to not only remove these harmful chemicals from water but also to effectively degrade or destroy them.
In a paper recently published in the Journal of Hazardous Materials, one of the leading journals in environmental engineering and pollutant remediation, Aich’s team outlined how the team used carbon-metal based nanomaterials to degrade the two major PFAS – perfluorooctanoic acid (PFOA) and perfluorooctanoic sulfonic acid (PFOS) – that are major targets of the new EPA regulations.
“We’re running against time,” said Aich, associate professor of civil and environmental engineering. “These chemicals don’t degrade in the environment and are present in water, air, and soil. We are all drinking water every day, and there’s a minimum amount (of PFAS) you can consume before it will start to affect your body.
“We can separate the PFAS from water with different types of filters. The problem, then, is disposing of the removed PFAS because there are not many effective technologies for destroying them and keeping them from re-entering the ecosystem.”
A team headed by Nebraska Engineering's Nirupam Aich is using carbon-based nanomaterials to degrade the two major PFAS that are the major targets of new EPA regulations.
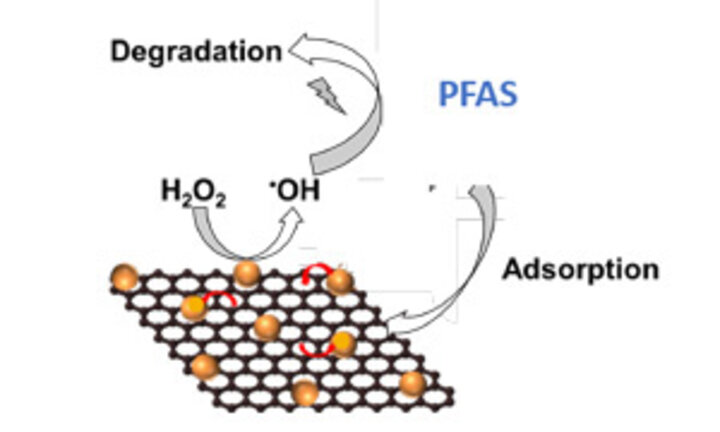
Aich’s team confirmed that their newly designed carbon-metal based nanomaterials – made from reduced graphene oxide (rGO), sheet-like carbon structures, and nano zero valent iron (nZVI) metal nanoparticles – can degrade PFOA and PFOS molecules and identified how this breaking of PFAS molecules leads to generation of other ions including fluoride, acetate, or formate.
In this process, the carbon-fluorine bonds in the chemicals are broken by a reactive process. In that catalytic reaction, these nanomaterials help create containing highly reactive molecules that can break the carbon-fluorine bonds and degrade the PFAS. The team further looked at how different chemical constituents of water such as pH or salt can affect the performance of this nanomaterial in breaking PFAS.
“The most common current technique to destroy PFAS is incineration, heating it to about 3,000 degrees Celsius,” said Aich, who began research on this path in 2018 while at the State University of New York (SUNY) at Buffalo, where some of the team members are still working. This research was supported by the National Institute of Health from the National Institute of Environmental Health Sciences (NIEHS) Superfund Research Program.
“But that’s highly energy-intensive and expensive and can create greenhouse gases. If we can create technology that breaks the chemical bonds and destroys the PFAS in other ways using nanomaterials, we can remove the PFAS in an environmentally safe manner. That’s what we are trying to do.”
The new EPA rules announced April 10 require municipal water utilities to monitor for PFAS for three years and to notify the public and reduce contamination if levels exceeded the new standard of four parts per trillion (PPT). The previous standard was 70 PPT. If those samples exceed the new standard, the utilities have two years to install equipment or filtration systems to meet the acceptable standard.
Nine days later, the EPA designated the two major PFAS – perfluorooctanoic acid (PFOA) and perfluorooctanoic sulfonic acid (PFOS) – as hazardous substances, thus requiring polluters to pay for any cleanup activities if required in any places.
Aich’s newly formed Nebraska Engineering research team is taking these PFAS-destroying nanomaterials and applying leading-edge technologies – such as 3D printing or membranes – to create filters that can take out PFAS. This includes two National Science Foundation-supported projects in which Nebraska graduate students are applying ultrathin layers of the nanomaterials on membrane surfaces.
Aich said technologies developed through his labs and elsewhere because of the new EPA mandates will eventually have a broader impact on Nebraskans, helping to control the forever chemicals that can affect drinking water and also leach into groundwater used to supply wells and irrigate agricultural crops.
And Nebraska’s environmental engineers are already among the leaders in developing new ways of combatting forever chemicals in the environment throughout the middle of the country,
“It won’t end with drinking water remediation. We will need similar technologies developed to remediate PFAS that can potentially be in biosolids used on farms and in smaller communities and rural areas that are not served by municipal water or waste treatment facilities,” Aich said.
“We are in a unique situation to help tackle these problems. We have experienced faculty at UNL who have been working in these areas for many years and are invested in finding solutions and helping Nebraska communities.”




